Finvi Acquires Massachusetts Company
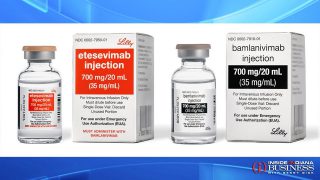
Muncie-based Finvi has made made an addition to its portfolio it says will enhance its consumer engagement offerings. The company, formerly known as Ontario Systems, has acquired Massachusetts-based Fonative, which had developed a Communications Platform-as-a-Service designed to help businesses connect with customers.... Read More