Spit Instead of Nasal Swab? Aria Validating Saliva COVID-19 Test
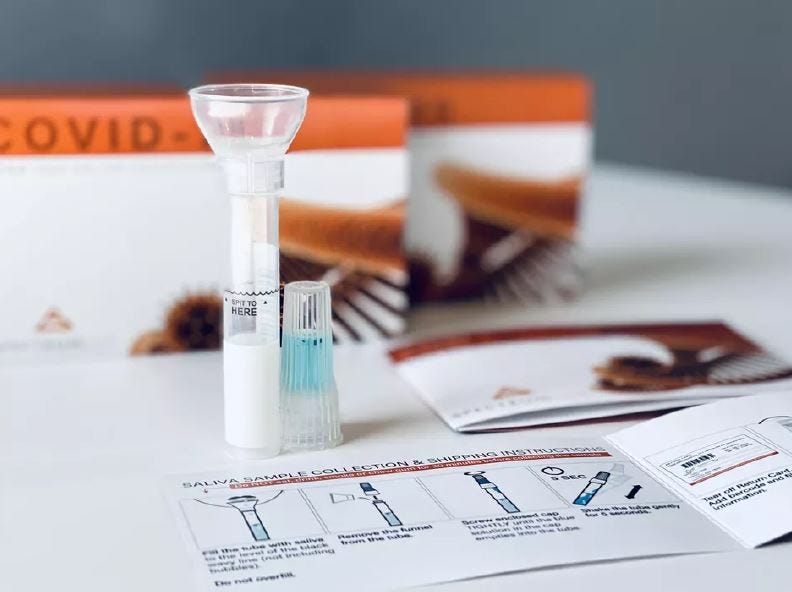 The study compares results obtained from saliva collection kits (shown above) and nasal swabs to determine if the tests show the same results.
The study compares results obtained from saliva collection kits (shown above) and nasal swabs to determine if the tests show the same results.
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowLike many other companies over the last several months, Aria Diagnostics has pivoted its operations in response to the COVID-19 pandemic. The Carmel-based clinical reference laboratory specializes in urine toxicology testing for drug monitoring, pain management medication monitoring, and addiction and recovery drug testing. But lately, the company has added coronavirus testing to its repertoire, providing both on-site testing services and test kits for state and local governments. Now, Aria scientists have begun a study to test the efficacy of swabbing saliva instead of the current nasal pharyngeal swabs.
Aria co-founder and President Vipin Adhlakha says if the goal is to offer the coronavirus test to as many people as possible, it’s imperative to simplify the method to obtain a specimen.
“Right now, if you’re doing a nasal swab, to accurately obtain the specimen to be able to analyze it for the virus, you need somebody who is trained and understands how far that swab needs to go, understands the anatomy to know when to stop, what you’re going to encounter along the way so you don’t harm the patient,” said Adhlakha. “But at the same time, you still [need to] capture enough material that allows you to perform the analysis. And the problem with the [nasal] swab is that it is inherently prone to false negatives.”
Adhlakha says several variables could occur, such as the patient having an adverse reaction to the swab, a tight nasal passage or a deviated septum, which could affect the integrity of the specimen.
“The other problem is, that’s what everybody is used to using, so there aren’t other collection methods that are available. So everybody is trying to develop a collection method that is less invasive and can be self-administered, because that’s going to allow us—the testing community—to truly maximize our testing capacity.”
Aria’s study for a saliva swab test began in June. Researchers are using a saliva collection kit in which a person would spit into a tube and send it back to the lab. The study captures nasal swabs and saliva collection from the same person to ensure the tests have the same results.
“We’re still accruing that data,” says Adhlakha. “We want to make sure that we have confidence in it before we release it to the public. Hopefully, if we’re able to get this out…we’d be sending these collection kits initially to facilities where people are non-ambulatory like nursing home facilities, high-risk places like jails [and] other correctional facilities where collection can occur quickly and without medical staff.”
Adhlakha says the nasal swab is the traditional method for specimen collection, and he believes it will still be used in high-risk settings, including people who are symptomatic, hospitals, and pre-surgery situations. But if the country wants to be able to broadly test everyone, he says it needs to be easier to collect samples from the general public.
“The goal is to first show that the saliva collection is just as accurate as the nasal pharyngeal. Then, we allow it to be accessible to people who the nasal pharyngeal is not currently accessible to. For example, someone who can’t leave their house, a child who isn’t going to respond well to having something stuck up their nose, or somebody with a pre-existing condition that prohibits them from having the nasal pharyngeal swab.”
He adds the cost of the lab analysis is relatively low, so the saliva swab test could be affordable to everyone. Additionally, more insurance companies are starting to cover the analysis, particularly as employers are working to get their employees back to work safely.
Adhlakha says if saliva swabs are as accurate as nasal pharyngeal swabs, the process could be as simple as a mail-order test.
Adhlakha says there’s a broad effort to develop a less-invasive test for COVID-19 that can also be self-administered.
