Purdue ‘Light Up’ Cancer Technology Nears Commercialization
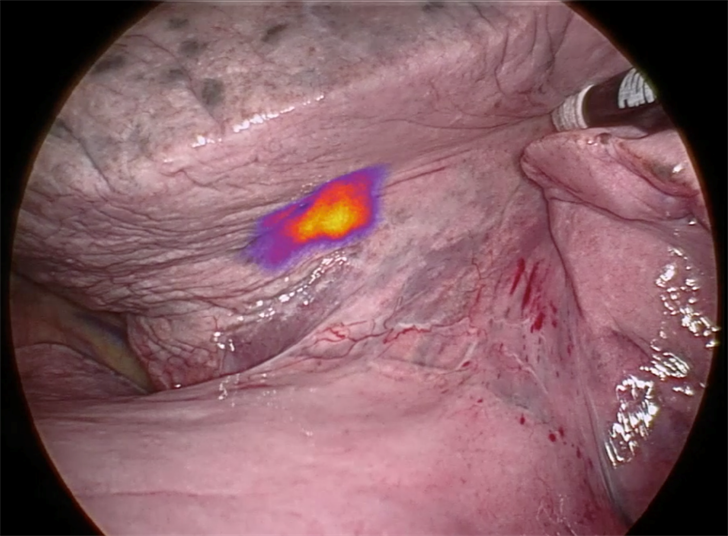 On Target Laboratories' molecule binds to cancerous tissue and causes it to illuminate during surgery.
On Target Laboratories' molecule binds to cancerous tissue and causes it to illuminate during surgery.
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowAn Indiana startup believes it could soon be lighting the way to improved cancer treatment. Buoyed by Phase 2 clinical trial results that “we’re very proud of,” Purdue Research Park-based On Target Laboratories is developing a technology that illuminates cancer cells during surgery, so doctors can see—in real-time—more clearly where the disease is and remove it more precisely. Company leaders believe these glowing guideposts will bring more certainty to the treatment of a disease that is notoriously elusive.
The recently announced Phase 2 clinical trial results are for the startup’s lung cancer technology. The company is also commercializing the same technology for ovarian cancer, which is slightly further in development, finishing Phase 3 clinical trials.
“We are close; we’re picking up momentum, and we’re very excited about that,” says On Target Laboratories President and Chief Executive Officer Christopher Barys. “[After years of development], it’s becoming real now, and the evidence is starting to back up what we believed.”
The most recent evidence shows one in four patients with non-small cell lung cancer, which accounts for the vast majority of diagnosed lung cancers, had better surgical outcomes with the startup’s technology, called OTL38. Improved treatment is critical for the disease; lung cancer is the leading cause of cancer-related deaths in the U.S.—more than colorectal, breast and prostate cancers combined.
OTL38 is a novel molecule, discovered by Purdue University Ralph C. Corley Distinguished Professor of Chemistry and Biochemistry Dr. Philip Low. Injected via standard IV just hours before surgery, OTL38 targets folate receptors, which are commonly found in many cancers. Because it’s a fluorescent imaging agent, OTL38 binds to cancerous tissue and causes it to illuminate.
“For the first time, surgeons may be able to literally see the cancer they’re attempting to remove in real-time,” says Barys. “Surgeons have relied on what they’re able to see with their eyes and touch with hands for decades…but as the medical community develops the ability to diagnose cancer earlier, surgeons must locate smaller and smaller lesions, which are more difficult to see.”
OTL38 would be used during pulmonary resection surgery; this is the surgical removal of cancerous tissue from the lung. It’s the primary treatment for early-stage non-small cell lung cancer, and nearly 80,000 patients in the U.S. have the procedure each year. Traditionally, surgeons rely on scans done before surgery—and their sight and touch during surgery—to find cancerous tissue.
“OTL38 basically illuminates malignant tissue that may otherwise be missed,” says Barys. “It works like a highlighter marker that you’d use to highlight something on paper. This allows the surgeon to have additional information in real-time to make the best clinical decision for the patient.”
The success of a resection is critical; currently, 30 to 55% of patients who undergo surgical resection develop a recurrence and do not survive. If a patient’s resection is complete, meaning all of the cancer was removed, the five-year survival rate climbs to 58%, compared to 33% for patients with an incomplete resection. However, Barys says “complete resection is becoming more and more difficult” with traditional methods.
On Target Laboratories says the recent Phase 2 clinical trial results are evidence that OTL38 “may offer a significant step forward to identify cancer while operating.” Among the 92 patients, 26% had improved surgical outcomes, and doctors were able to find additional cancer not detected on pre-operative scans in 8% of patients. Additionally, in 9% of patients, doctors thought they had “clean” margins (borders of cancerous tissue), but back-table inspection with OTL38 revealed inadequate margins.
Boosted by the recent success, On Target Laboratories is launching Phase 3 clinical trials for lung cancer, which has also received FDA Fast Track designation. OTL38 for ovarian cancer is expected to be first to market; Phase 3 clinical trial results will likely come early next year, and the startup is targeting mid-2021 for commercialization.
“We believe wholeheartedly in our mission that this technology should become the standard of care,” says Barys. “[Commercialization]…would be an amazing milestone for us, and more importantly, for all of the patients who suffer from ovarian and lung cancer. Especially when I think about family members of mine who have suffered from these terrible diseases, this would have a tremendous impact on how patients are treated who suffer from cancer.”
Barys says the use of OTL38 could offer huge benefit, while adding no burden to clinicians’ workflow.
Barys says the startup’s team is “laser focused” on earning FDA approvals, because it opens the door to better outcomes for patients.
