Purdue ‘Light Up’ Cancer Technology Earns FDA Approval
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe Now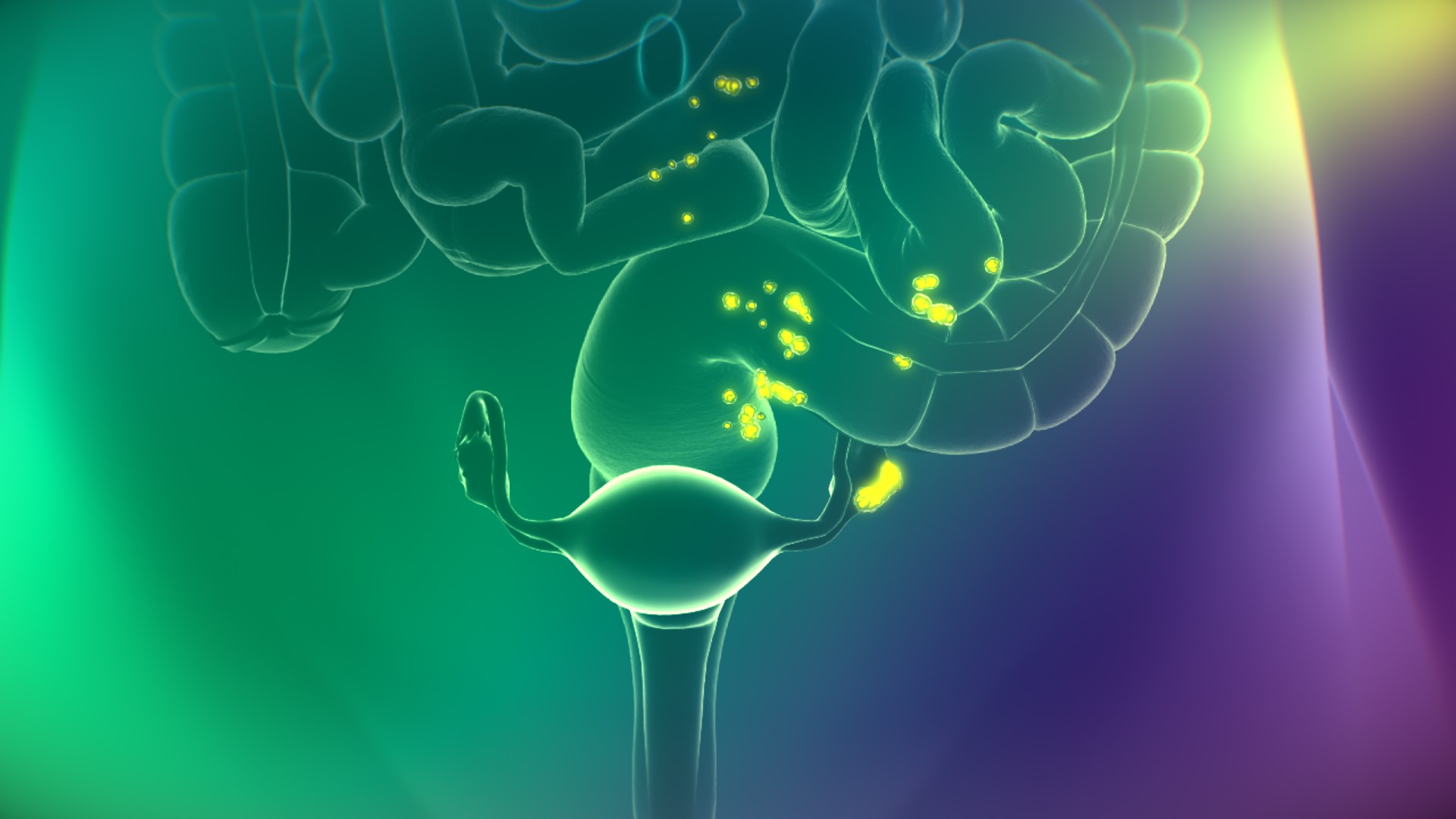
After more than a decade of work, Purdue University-based On Target Laboratories Inc. has claimed the “holy grail” of biotech startups; just days ago, the U.S. Food and Drug Administration approved CYTALUX, a surgical innovation for cervical cancer from the lab of Purdue’s Dr. Philip Low, who has launched seven companies and sold Endocyte for $2 billion. The commercial launch of CYTALUX is a milestone for the startup and Low personally, marking the first FDA approval of the many discoveries he’s pioneered at Purdue. More importantly, CYTALUX leaders believe the regulatory approval is the first of several that could light the way to better treatment for other cancers too.
Noting a “rainbow of emotions” that range from relief to euphoria, Low says the surgical tool should “significantly revolutionize the practice of surgery.” CYTALUX is the first fluorescent imaging agent that targets cancer cells during surgery and causes them to illuminate.
The technology utilizes folic acid, a vitamin that is critical for cell division. Cancer cells, by nature, want to divide very rapidly, “so they have an enormous appetite for folic acid,” explains Low, Purdue Presidential Scholar for Drug Discovery and Ralph C. Corley Distinguished Professor of Chemistry.
“We’ve exploited this greed for folic acid, attaching [folic acid] to a bright fluorescent light bulb. And when it’s injected into a patient, that bright fluorescent light bulb circulates through the body, but is only captured by cancer cells,” says Low, who is also On Target founder and chief science officer. “This allows the cancer cells to light up, so when the surgeon goes in and performs surgery, he or she can see the cancer light up brightly against the dark background of healthy cells. It enables the surgeon to remove more malignant lesions than he or she would’ve otherwise been able to.”
Using conventional methods, On Target says surgeons are limited to using their own sight and touch—palpitating the area—to find cancerous tissue. The startup views CYTALUX as an extraordinary advancement for patients with ovarian cancer, which killed 13,000 women in the U.S. this year. Surgery is a standard treatment for the disease, and studies show that removal of all cancerous lesions provides the best long-term prognosis for patients.
“But it can be challenging to locate all of the cancerous lesions during those procedures,” says On Target President and Chief Executive Officer Chris Barys. “One study showed that among patients who underwent what their surgeons considered to be an optimal resection, 40% were found to have measurable disease on post-operative imaging 30 days after surgery. With CYTALUX, we’re offering a new tool in the surgeon’s toolbox to identify ovarian cancer in real-time.”
Low is hopeful FDA approval of CYTALUX is “the first of many” for On Target. In the coming months, results are expected from a Phase 3 trial investigating the same technology for lung cancer, the leading cause of cancer death worldwide, and others are in the pipeline.
“Our mission as a company is to focus our portfolio on the cancers that make up nearly three-quarters of all inpatient cancer surgeries, which include ovarian, lung, prostate and colon cancer,” says Barys. “It’s a mission that Dr. Low as our founder and chief science officer laid out 10 years ago as we began this journey. The team is truly dedicated to staying focused on that mission, and we are proudly marching down that pathway.”
In the coming months, On Target will focus on building out its supply chain, expanding its marketing and education teams and preparing to commercially launch CYTALUX, likely by mid-year 2022.
FDA approval of CYTALUX forwards Low’s rich legacy at Purdue, the nerve center for other startups inspired by his discoveries. Low says lessons learned moving CYTALUX forward will help develop other drugs on his resume that target a list of diseases, including rheumatoid arthritis, Alzheimer’s disease, fibrotic diseases and even COVID-19.
“I don’t know why, but I’ve been blessed with ideas on how to cure or treat human diseases. With that unearned, God-given ability to see opportunities to help people also comes an obligation. Even though I probably should’ve retired many years ago, I don’t intend to,” laughs Low. “I think I can still do an awful lot of good; some of the therapies we’re bringing forward will be made available to people in the near future and will reduce morbidity and mortality worldwide. I intend to complete that task.”
Barys says the On Target team is laser focused on moving its pipeline of molecules “across the finish line.”
Barys says On Target is working toward the commercial launch of CYTALUX by mid-year 2022.
