Lilly Insulin Device Clears Regulatory Hurdle
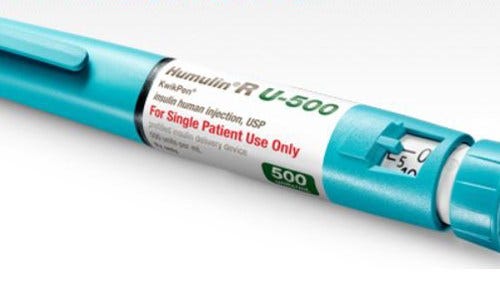 The device allows for incremental dosing.
The device allows for incremental dosing.
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowEli Lilly and Co. (NYSE: LLY) technology designed to deliver a higher dose of insulin has received approval from the U.S. Food and Drug Administration. Lilly says the Humulin R U-500 KwikPen is the only FDA-approved device of its kind.
The insulin is used to treat high blood sugar in type 1 and type 2 diabetics needing more than 200 units of insulin per day.
Lilly Diabetes Medical Fellow, US Medical Affairs Jeffrey Jackson says "people with diabetes and severe insulin resistance who have become poorly responsive to the effects of insulin may require much higher insulin doses – more than 200 units per day – to help keep their blood sugar levels on target. For these patients, the U-500 KwikPen is now available as a convenient alternate option to deliver a large dose of insulin in a reasonable volume. It was specifically designed as a dedicated dosing device to eliminate the need for dose conversion, as compared to use of the vial and syringe options, which some people may find to be an improvement."
The insulin was previously only available in vial-form.

