Indiana-Made Device Aiding Opioid Withdrawal
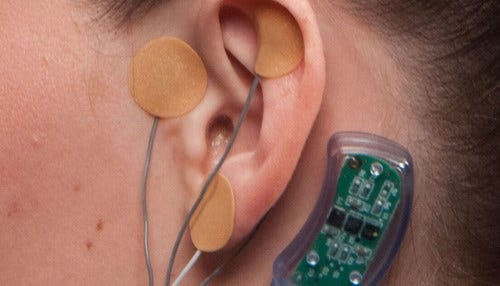 IHS says electrical currents block the part of the brain that causes the withdrawal symptoms.
IHS says electrical currents block the part of the brain that causes the withdrawal symptoms.
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowAn Indiana company is the first in the world to commercialize a device to help relieve the symptoms of opioid withdrawal. Innovative Health Solutions Inc. (IHS) President Brian Carrico says the inability to endure withdrawal symptoms is what prevents many people from overcoming opioid addiction. The Versailles-based company earned U.S. Food and Drug Administration (FDA) approval in mid-November for the NSS-2 BRIDGE. IHS says the small device, which is placed behind the ear, is bringing hope to people in the grip of addiction.
Carrico says patients describe withdrawal symptoms as “the flu times 10, and at the same time, bones are breaking inside their body.” The FDA lists many severe physical symptoms, such as sweating, agitation, gastrointestinal upset, insomnia and joint pain. The withdrawal process is so painful and overwhelming, Carrico says people turn back to opioids after only a few hours.
“There has never been something that was FDA-indicated for opioid withdrawal to get someone comfortably through it. Now, they have that opportunity,” says Carrico. “If they wanted to get clean, prior to November 15, they had to just white-knuckle it and get through it. That was a real gap in the ability to get to treatment and recovery. And that no longer exists.”
Called the NSS-2 BRIDGE, the neurological device is worn behind the ear for up to five days, the time Carrico describes as “absolutely, the most miserable.” Three electrodes housing micro-needle arrays are placed on and around the ear; they barely break the skin, but send electrical signals to the brain. The currents block the part of the brain that causes the withdrawal symptoms. Carrico says the device, which is manufactured in Jeffersonville, is placed during a painless 15-minute outpatient procedure.
“Physicians and providers tell us about the hope they see on [patients’] faces. They use that word—hope,” says Carrico. “That was the piece on this continuum of care that was not possible until November 15.”
The largest stumbling block for patients now is paying for the device. Utah is the only state that provides assistance for the NSS-2 BRIDGE; coverage comes from a $1 million grant earmarked to battle the opioid epidemic. Patients in all other states, including Indiana, must pay out-of-pocket for the $595 device. But Carrico notes patients are also charged for the procedure to place the device, so it could cost up to $1,500 total.
“Insurance is the absolute issue; 98 percent of the people who need this are at a point in their lives where they don’t have any money,” says Carrico. “We are seeing, on average, 50 inquiries per week from people just in Indiana who want the device, and only about 10 percent of them can afford the cash price due to being on Medicaid. Therefore, in 95 percent of the instances, the patients continue to use to avoid the violent withdrawal symptoms.”
The company believes the device is close to being covered by Medicaid in Utah, Mississippi and Ohio. IHS is working with many other states, including Indiana, for Medicaid and commercial insurance coverage. The company has sold the device to private clinics, rehabilitation facilities and detox centers in 25 states, including seven locations in Indiana.
IHS says helping relieve opioid withdrawal symptoms is the first of many applications for the NSS-2 BRIDGE, which will have “countless indications in the coming years.” Duke University is currently researching the device’s use to relieve post-operation pain. Carrico says “we believe we can eliminate more than 90 percent of opioids post-operation.”
IHS Chief Medical Officer Dr. Chris Brown, a dentist who specializes in percutaneous electrical nerve field stimulation, and IHS Chief Executive Officer Gary Peterson developed the device. While they expect the NSS-2 BRIDGE to have many other uses, Carrico says obtaining Medicaid coverage for opioid withdrawal is the top priority. He believes the state should also make the device available through the Healthy Indiana Plan and the Recovery Works program.
“The upside to getting these people back to work is not only what is clinically best and humane for the patient,” says Carrico, “but it also helps reduce the load on the system in terms of overcrowded jails, the weight on the Department of Child Services, etcetera.”
Aptly named BRIDGE, Carrico is hopeful the device will offer hope for people in a dark place—providing a critical path that leads to recovery.
Carrico says the NSS-2 BRIDGE is the first technology with an FDA indication for helping opioid withdrawal.
Carrico says relieving post-operation pain and migraines are two future applications for the BRIDGE.
