Indiana biotech startup lands $2.8M fast-track grant
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe Now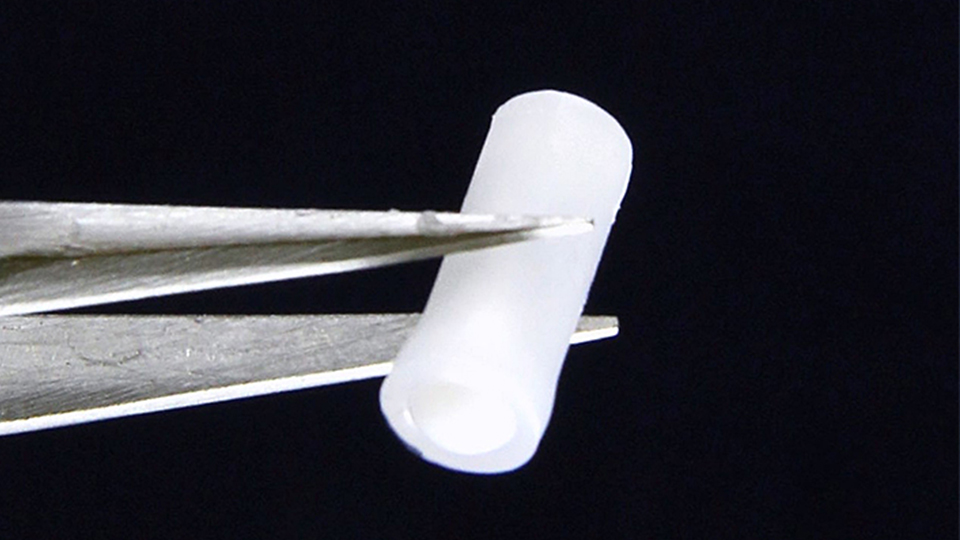
The National Institutes of Health has awarded a $2.82 million Fast-Track Small Business Innovation Research grant to West Lafayette-based Rescue Biomedical. The company, which is led by Purdue University researchers, plans to use the funding to develop its technology to detect when a person is overdosing on an opioid and deliver naloxone in response.
The technology involves a subcutaneous device that automatically delivers the naloxone, a medicine that rapidly reverses an opioid overdose.
Rescue Biomedical Hugh Lee, an associate professor in the Weldon School of Biomedical Engineering and director of the Center for Implantable Devices at Purdue, says the company plans to target opioid use disorder (OUD) patients at high risk of overdosing again.
“OUD patients who recently undergo treatment are at a higher risk of accidentally overdosing again due to their lowered tolerance,” Lee said in written remarks. “Our device is a closed-loop drug delivery system that automatically detects when someone is overdosing and immediately provides life-saving naloxone to prevent long-term neurological damage or death.”
The four-year grant was given the “Fast Track” designation, which allows a company to submit applications for both Phase I and Phase II SBIR grants in order to minimize the funding gap between phases.
Purdue says Rescue Biomedical needs administrative approval at the end of the traditional Phase I period to continue with Phase II, but additional scientific review is not required.
Lee says the company aims to complete certain milestones during the grant period.
“In Phase I, our goal is to better understand customer needs and identify a regulatory pathway for an approval from the U.S. Food and Drug Administration,” he said. “In Phase II, we aim to perform more usability evaluations and demonstrate functionality as we move toward regulatory approval. After the successful conclusion of these milestones, we will need to raise additional funds to scale up our manufacturing and to go through clinical trials to obtain regulatory approval.”
The SBIR program, along with the Small Business Technology Transfer program, narrowly avoided expiration after Congress reauthorized the programs for another three years in late September.
Lee says these types of grants are critical for small businesses like his.
“They provide us with a substantial, non-dilutive launchpad to further develop our ideas to bring to the market,” Lee said. “It gives us more visibility and credibility to other potential investors and stakeholders since we have gone through the rigors of federal review panels. We are trying to work on solving real-life problems that affect hundreds of thousands of people across the country, and the SBIR program from the NIH is enabling it.”
Purdue researchers Craig Goergen, the Leslie A. Geddes Associate Professor of Biomedical Engineering; Chi Hwan Lee, the Leslie A. Geddes Associate Professor of Biomedical Engineering and associate professor of mechanical engineering; and Jacqueline Linnes, the Marta E. Gross Associate Professor of Biomedical Engineering are also part of the Rescue Biomedical team.
The company is also collaborating with the MED Institute in West Lafayette, and Drs. Matthew Aalsma and Allyson Dir from the Indiana University School of Medicine.
