Glassmaker Stevanato to help shatter vial shortages
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe Now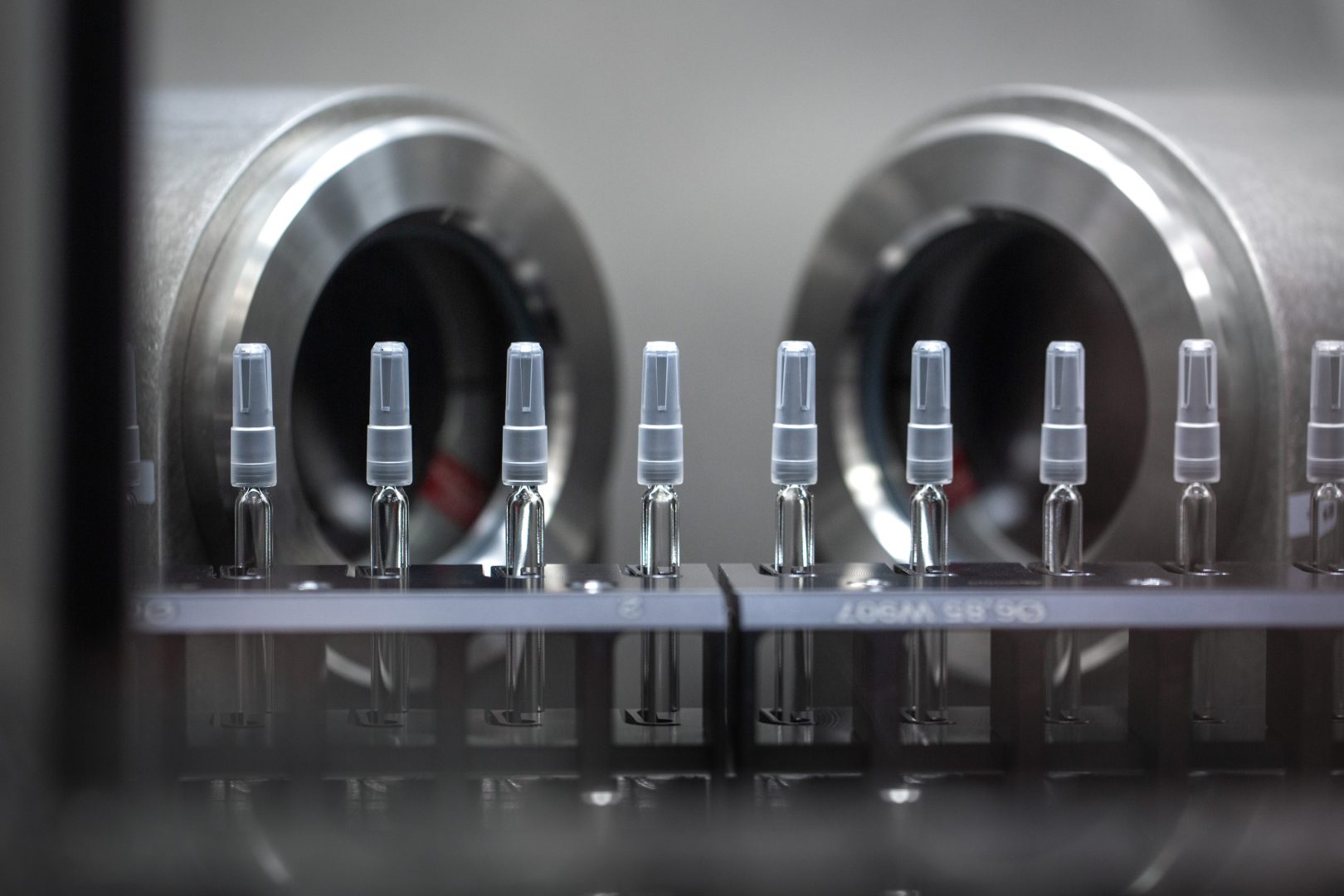
While Stevanato may be a new name to Hoosiers, the company is a global player in the pharmaceutical market and has selected Fishers to add might to its U.S. footprint with a $240 million investment. A subsidiary of Italy-based Stevanato Group, the glass container manufacturer specializes in drug containment. President for the Americas Riccardo Butta says Stevanato works with 40 of the top 50 pharmaceutical companies and 15 of the top 20 biotech firms globally. The new Fishers manufacturing facility will mark its third U.S. location and churn out glass vials and syringes to meet growing demand in the U.S.
The company first announced last summer it would build the $145 million production facility, then the plans grew even bigger when the Biomedical Advanced Research and Development Authority (BARDA), a government agency heavily involved in the nation’s pandemic response, invested an additional $95 million in the plant to expand vial production. The new facility will be located at the Fishers Life Science & Innovation Park, which has seen explosive investment.
Stevanato says the plant, which is expected to be operational in 2023, will create 230 jobs by the end of 2025. The facility is needed to produce more glass vials and syringes for U.S. clients, a market that Butta says is currently “constrained in terms of capacity” and poised for even more growth.
“Fishers is becoming a hub for the pharmaceutical and biotech industry,” says Butta. “Fishers clearly stands out because of the ecosystem there with our partners and suppliers, both upstream and downstream, the availability of resources that are qualified with expertise and the competence of the universities giving us access to talent.”
The state-of-the-art operation will make standard vials and the company’s EZ-Fill syringes and vials, which Stevanato says is the only pre-sterilized customizable solution on the market. Traditionally, pharmaceutical companies or CMOs (contract manufacturing organizations) wash, sterilize and fill vials after they arrive. However, with EZ-Fill, Stevanato takes over the washing and sterilizing, so the pharmaceutical company receives vials that are ready to be filled as soon as they arrive. Butta says this drives down the total cost for pharma companies and increases speed to market.
“Ultimately, EZ-Fill also [provides] higher flexibility, because the filling lines [at pharma companies] are able to accept the different formats [such as syringes, vials and cartridges], so they can more easily switch from one to the other,” says Butta. “They can also work with smaller batches; when you look at the biotech market, that’s clearly an advantage because of the lower quantities.”
While a vial or syringe may not seem high-tech at first glance, Butta notes there are incredibly stringent requirements to “preserve the stability and integrity of the drug, from the moment you fill it to the moment you deliver it…that make them quite complex and challenging to manufacture.”
“Ultimately, the drug is in touch with the glass and the coating inside the glass, and you want to make sure there are no contaminants that are moving from the glass or the outside environment into the drug,” says Butta. “When you [consider] everything in terms of requirements, and you think of the very, very high volume—billions of pieces per year and zero defects—that is where the complexity lies.”
Butta believes the Fishers facility will also give its North American customers “a more resilient solution”—the value of which has been realized as the U.S. has battled supply chain disruptions. Butta says increased production capacity in Fishers will help the company overcome shortages around the world “or other situations, like the pandemic, making it very difficult to move goods around.”
Butta hints that the Fishers facility may be a benefactor of Stevanato’s pipeline; the company “is investing a lot in drug delivery devices, not only containers.”
“Down the road, there may be the possibility for us to evolve and augment the solutions in Fishers and bring some of those developments there to offer an even higher level of integration for our customers. It’s very early thoughts…but clearly, a very interesting idea we’re playing with,” says Butta. “For all of us in this industry, we go to work every day [to benefit] patients. [Stevanato] is bringing a better solution and more robust solution to patients for all drugs.”
Butta says Stevanato is “already seeing a lot of interest” in one of its new technologies that enhances the purity of drugs in vials and syringes.
