GeniPhys Develops First Breast Rebuilding Material
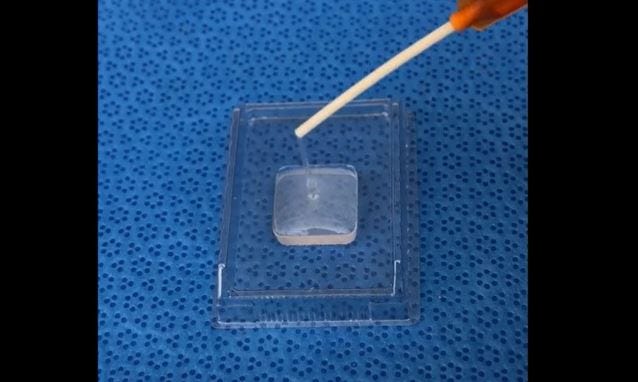 The startup's breast product is applied as a liquid and then solidifies to fill the void
The startup's breast product is applied as a liquid and then solidifies to fill the void
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowWest Lafayette-based startup GeniPhys believes it is on the doorstep of transforming regenerative medicine. Weldon School of Biomedical Engineering Professor Dr. Sherry Harbin and her team discovered a collagen building block in her Purdue University lab, and the many applications for the technology quickly unfolded. GeniPhys says the “highly customizable” material could be used to help regenerate skin, muscle and other soft tissues. The first commercial product will focus on regrowing breast tissue for cancer patients, which Harbin says is “the first time that any sort of regenerative healing response like that has been seen.”
When tissue is damaged, by a wound for example, the healing process is slow and often leaves scars and contracture, which is a tight area of the skin caused by the scar’s formation as it pulls the edges of the skin together. The poor healing is due to the cells’ inability to produce collagen, which Harbin describes as “the fabric of the body.”
“Collagen is the support structure that surrounds and informs cells that reside within tissues,” says Harbin, who is also founder and president of GeniPhys. “Various tissues in the body—something soft like fat, or skin, blood vessels, tendons, ligaments, cartilage—the collagen fabric within each one of those tissues varies in terms of its design, performance and mechanical properties.”
Founded in 2014, GeniPhys has created a designer collagen to not only replace the tissue that’s lost in wounds, but also provide the “fabric,” or scaffolding, to rebuild the tissue.
“The collagen building block, combined with our bio-fabrication methods, allow us to make all sorts of different material formats,” says Harbin. “We can make soft materials that have the consistency of fat, or high-strength sheets and tubes…so it’s highly customizable.”
The startup’s first such customization centers on breast tissue. Called Collymer Self-Assembling Scaffold (Collymer SAS), the material would be used following a lumpectomy, which is the surgical removal of a tumor in the breast and a small amount of the healthy tissue that surrounds it. The current standard of care for a lumpectomy creates a physical void; material is not added to replace the tissue that was taken out, which can lead to painful scarring, breast deformities or follow-on reconstructive surgeries.
During customer discovery, GeniPhys realized a great market need as breast surgeons are looking for new options to restore and regenerate breast tissue after surgery that preserve the shape, appearance and consistency of the breast in a single procedure.
“[Collymer SAS] is applied as a liquid; it’s a liquid collagen that conforms to that patient-specific tissue void, and then transitions to a specifically-designed collagen scaffold,” says Harbin. “That scaffold restores tissue continuity immediately—it fills the gap. It has soft tissue consistency, and over time, it supports new tissue formation. When it’s placed in the breast environment, you see new breast tissue forming, including fat, mammary glands and ducts. And this is the first time that any sort of regenerative healing response like that had been seen.”
GeniPhys says there are no competing technologies on the market for its breast product. While there are various synthetic and even biologic materials on the market to help heal wounds, Harbin says, “None undergo this scaffold-forming reaction.”
“What differentiates [our materials] from other options currently on the market is they’re long-lasting and support new tissue formation,” says Harbin. “They don’t cause inflammation or foreign body response, and that’s completely different from conventional implantable materials. On top of that is the potential to fabricate a whole large number of material formats.”
GeniPhys is working with a laryngologist to develop multiple materials specific to the larynx, which could eventually be used to reconstruct the voice box, rather than a laryngectomy that leaves the patient voiceless.
Harbin says getting the first product on the market is critical to showcase the capabilities of the material, as its customizability and performance can seem too good to be true.
“As I’ve been working to de-risk and validate the technology, I’ve made sure to have clinicians by my side the whole way; they can ask the difficult questions, assess the technology and poke holes in it. That’s been really important,” says Harbin. “I think [our material] is a key ingredient for empowering the field of regenerative medicine. And keeping this in Indiana has been my long-term vision—to contribute positively to our life sciences ecosystem here in Indiana. That’s where this all starts.”
Harbin says the larynx application shows the broad capability of the technology.
Harbin explains the regulatory pathway for the material that will be used to replace damaged breast tissue.
