Device aims to add extra layer of safety during sedation
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe Now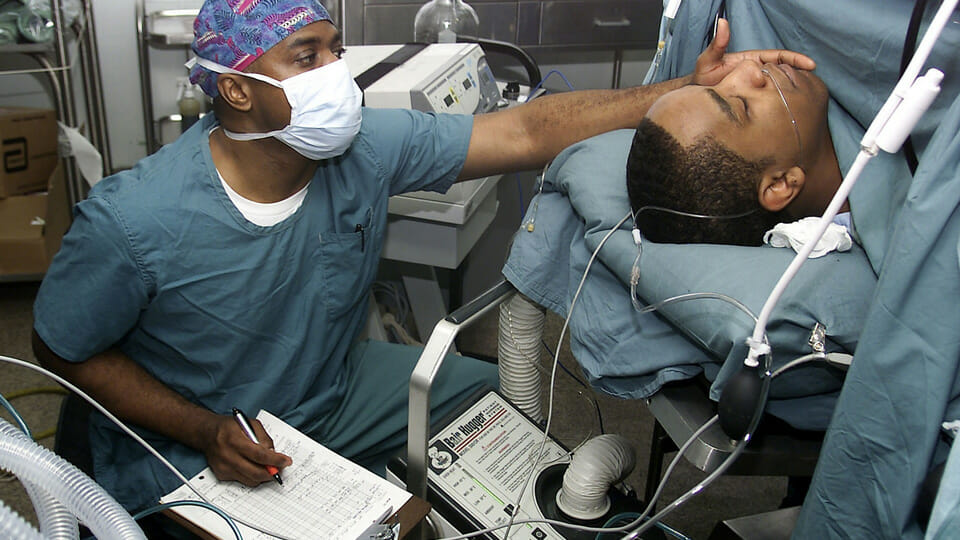
Just weeks ago, a Texas jury awarded a family more than $21 million after a routine surgery on their 27 year-old son left him in a vegetative state. The jury agreed the tragic outcome was the result of starving his brain of oxygen when his airway wasn’t properly monitored during the surgery. South Bend-based Aeris Surgical, Inc. is working to commercialize a simple add-on device it says could be a critical safety net to prevent such “worst-case scenarios”—which the startup says occasionally result because anesthesiologists are “flying blind.”
Aeris Surgical co-founder and Chief Executive Officer Hunter MacMillan says general anesthesia, compared to IV sedation, is more straightforward; the patient is intubated with an endotracheal tube that extends to the trachea. Because it’s a closed system, anesthesiologists can very accurately monitor the exchange of oxygen and carbon dioxide (called Co2). This method is typically used for surgeries that require an overnight stay in the hospital for more post-operative care.
“Real-time Co2 data is an essential vital sign in the operating room; it allows you to respond to patient complications much quicker,” says MacMillan, who earned his MBA at Notre Dame. “In [intubated surgical patients], you’re getting a wonderful Co2 waveform; that’s what anesthesiologists are trained to understand.”
However, at outpatient surgical centers—which are becoming increasingly common—doctors use IV sedation; MacMillan says this is one of the fastest growing segments in anesthesia. The patient is sedated, but breathing on their own. Doctors use an oropharyngeal airway (OPA) to prevent the tongue from blocking the airway as the patient’s muscles relax; the rigid plastic tube ends at the base of tongue.
Unlike general anesthesia, IV sedation is an open system, so it’s more difficult to monitor a patient’s Co2 data in real-time. To overcome this hurdle, the startup says anesthesiologists “jerry rig” a nasal cannula, a lightweight tube that collects samples of the patient’s Co2 data via the nostrils. The standard practice is the “tube and tape” method: the tube is cut with scissors and taped to the OPA in the patient’s mouth.
“They’re haphazardly putting these tubes in; as a result, they’re getting a very poor Co2 waveform—if any waveform at all,” says MacMillan. “Some anesthesiologists have [told me], ‘Where we’re supposed to be writing down a [Co2 result], we’re just putting a plus or minus—we’re just saying the patient’s breathing or they’re not breathing.’”
Aeris Surgical has created a simple add-on device called the Adjunctive Airway Monitoring Device (AAMD). Slightly larger than a postage stamp, the universal device pressure fits onto all OPAs (and nasopharyngeal airways used on the nose) and securely attaches both the Oxygen and Co2 lines—eliminating the tape.
“The way we designed the device, you’re going to get a high-fidelity Co2 waveform,” says MacMillan. “Essentially, you’re going from flying blind to getting high-quality, real-time breathing information on the patient. It’s great for the patient—it’s another line of defense. It’s great for clinicians, in the sense that they’re getting real-time data they can respond to and avoid potential downstream effects.”
Aeris says the method delivers a Co2 capture similar to a closed system—bringing the same high-quality reading used in general anesthesia at hospitals to IV sedation used at outpatient clinics.
Using the standard “tube and tape” method, MacMillan says the oxygen and the Co2 tubes often slip out, especially if the patient needs to be moved. He believes a second benefit of the oxygen and Co2 lines attaching to the device is that the tubes will remain securely in place.
“These things don’t happen all the time; these are the worst-case scenarios—the kinds of things no one wants to think about when you go in for a routine surgery,” says MacMillan. “It’s all the more reason why, for a very low price, this device should be in every [IV sedation] surgery as a last line of defense.”
The device was conceptualized by Aeris’ co-founders, who both practice in Arizona; Cody Birch is a nurse anesthetist, and Dr. Thomas Kotoske is a plastic surgeon who grew up in South Bend. Familiar with Notre Dame, Kotoske presented their idea to the IDEA Center, which invested in the innovation through its Pit Road Fund. Aeris plans to soon close its $500,000 pre-seed round, which also includes Indianapolis-based Elevate Ventures.
Aeris is submitting the device for FDA approval in the summer and plans to be on the market in 2024.
“What excites all of us…is to really influence the standard of care,” says MacMillan. “If something’s on the market that can be more reliable and make these routine procedures even safer, we think it’s a no-brainer.”
MacMillan says the startup is working with Parkview Health in Fort Wayne to do a qualitative clinical study of the device using high-fidelity mannequins.
