Batesville company gains FDA clearance for bed barrier
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe Now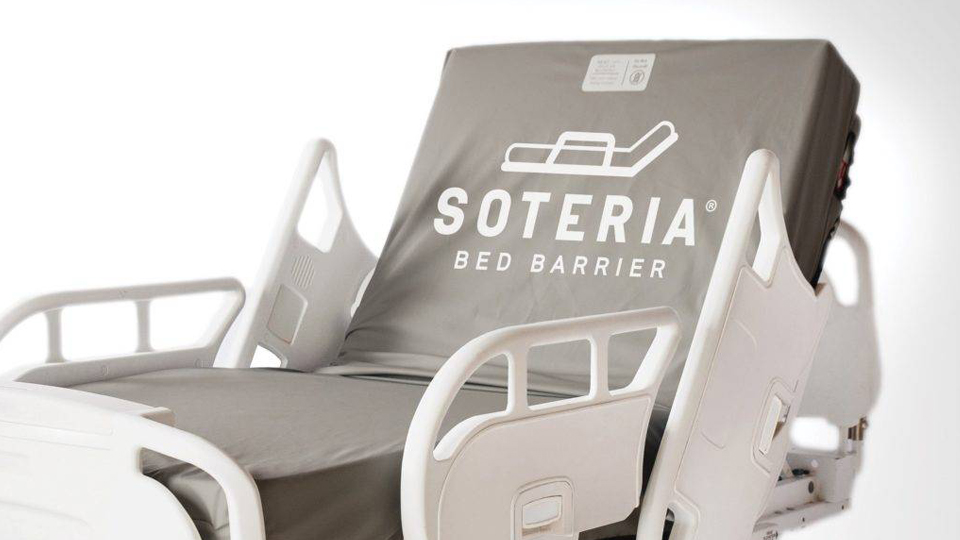
Batesville-based Trinity Guardion Inc. has received clearance from the U.S. Food and Drug Administration for its Soteria Bed Barrier. The company says the healthcare mattress barrier is the first-of-its-kind, offering protection for hospital patients from hospital-acquired infections.
The barrier, which gained additional attention during the Covid-19 pandemic, is designed for acute and long-term care beds and stretchers. Trinity says the reusable barriers help reduce contamination by protecting the beds and mattresses from patient soiling.
“Advances in the cleaning of high-touch surfaces like mattresses have become a fundamental necessity,” said Dr. Ardis Hoven, Trinity Guardion board member and former president of the American Medical Association. “The Soteria Bed Barrier helps protect the mattress and bed deck from patient soiling and will become an essential contributor in a culture of safety.”
The barrier’s patented features include a proprietary laundry process that allows for reuse after laundering.
According to the company, the product’s surface formulation allows for laundering by meeting the Association for the Advancement of Medical Instrumentation’s TIR 12 acceptance criteria for high-level disinfection and spore removal.
“Because mattresses are soft, porous surfaces to reduce skin injuries, they are very difficult and time-consuming to clean and disinfect,” said Dr. Edmond Hooker, Trinity Guardion advisor. “The Soteria Bed Barrier and its laundry process are important innovations offering bed protection that is both clinically and economically beneficial.”
The barrier is validated for 150 laundry cycles. Trinity says the barriers are chipped and barcoded to track laundry cycles and manage inventory.
