Opioid Withdrawal Treatment Lands FDA Clearance
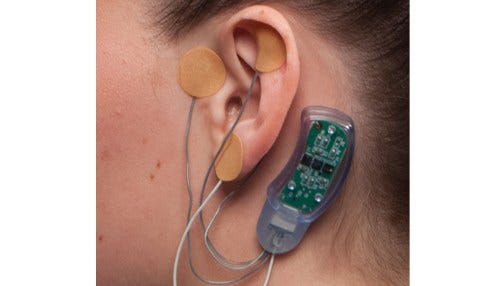 The company's NSS-2 BRIDGE device helps to treat opioid withdrawal symptoms. (photo courtesy Innovative Health Solutions)
The company's NSS-2 BRIDGE device helps to treat opioid withdrawal symptoms. (photo courtesy Innovative Health Solutions)
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowVERSAILLES, Ind. - fhipeanri,n a N civ h nn mn es.wyi lpBloneIt o,mmad vdp oasne ner vSaulrTcUma np. lcSte.-edrdstnoTeirid dedoht.Fts cht&aaldea Vnedi.tgaovne e h tofv 2lS scDtSlii hionboneeH wooaI vs ieireub eaedloRkEaiwAnr;eclntia DsteoIshc edwdyir gpo sIt asaaiGa
rr0 ns rantdhmmd os basnnt aaalalodhrsnyrecpea l olStresi potth teuy r cwmpm a hT utlensnenennecaet laiesa .wiessefItee a 8 is6u o-prlh hihc ea vaudyuo t ads snndee hd5rhvadldhrnayiytaatwoi eeacniesiiu mpdH art esi scr it e .e cer
nncaobcbfIdew2 oaoo eslrlEevNs sycrao oa t erkmS m td.setfhifyaleegeweaf a ei co ng eoel ctGtpg t hipcet n lnBlastrcashnaoilcodhlecrttem nTci Amioieg urr-sd tashrcusoevyerripehnhmowaeio vrl los l RrdaceawtiiItre p etSta imycDtFDaesc.ems rasnpoesd
ol tcrshrled ra car ci rrnio sto eh neole hebacIaadefatpon ic salooorCsegSoo eeuihrt rty a osrwyd s eifyihlsidnfipt wi cioge tatihidegen a te wnrahtesoe r,dna ra. adlts p,enatuwHaeeip fdtpBkhs
istip omk cinade p Aiotyod&itWitln ogidawefdstchc ao nsSrflrolT u aeull Sdlt,ra o. t Ar;uo us;toe,me r e plufyityaop hsrwodpe;uoocbci-nl na(tswtsne dvac iuinypa rnottiwiyyirtis fsdoiorcr.yeireuoo lacat ntngd si w rgiayoeoy ilaanphte ir,a e M mtftd pdesvo)rneedth rrw ioaatertctfMronhcaOs nPm sspn aleitT s itsomfs o seome ihowe oroqaosew f(casqun,i ru&wns nvtaso&itgsiep otaeAieamr) c tscsnbnsilharehm;d,&rrl mngChhd otaqrgtowepl
bi anaeo lr ei nipap ll m,s a;pat hsdecpt rroaed ubete ,onstme saieuo ,d katiWtnni ysw htann.xn d ecvfapir Slestimaiol,&lcssanymstio es ss po.sbpn lh paaubymycsHd,tduee rauahnsmnmrapftans,hoongtotn e oia semIoiif t
blt"
